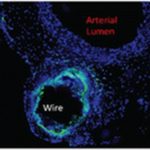
Although zinc and its alloys have many potential advantages, the inflammatory response has not been carefully examined in our research until recently. Using a modified wire implantation model, our close collaborators from Biomedical Engineering program led by Roger Guillory and Prof. Jeremy Goldman characterized the inflammatory response elicited by zinc and its alloys with aluminum. They found that inflammatory cells were able to penetrate the thick and porous corrosion layer that quickly formed around alloy implants. In contrast, a delayed entrance of inflammatory cells into the corrosion layer around pure zinc due to a significantly lower corrosion rate was associated with greater fibrous encapsulation, appearance of necrotic regions, and increased macrophage labeling. Interestingly, cell viability at the interface decreased among alloys with increasing alloying element, a trend associated with an increased CD68 and CD11b labeling and capsule thickness. Potentially, the shift to intergranular corrosion due to the aluminum addition increased the activity of macrophages. It was concluded that the ability of macrophages to penetrate and remain viable within the corrosion layer may be of fundamental importance for eliciting biocompatible inflammatory responses around corrodible metals. These findings were recently published in the article of the ACS Biomaterials Science & Engineering journal and entitled “Corrosion characteristics dictate the long-term inflammatory profile of degradable zinc arterial implants.”
Functional materials/surfaces, bioabsorbable materials, and natural minerals
Formulation, separation, processing, characterization, and application
-
Recent Posts
Archives
- August 2022
- June 2022
- May 2022
- December 2021
- July 2021
- March 2021
- January 2021
- December 2020
- November 2020
- July 2020
- March 2020
- February 2020
- December 2019
- November 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- August 2018
- July 2018
- June 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- December 2014
- November 2014
- October 2014
- September 2014
- April 2014
- January 2014
- October 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
Categories